arsenic removal
WHAT IS ARSENIC CONTAMINATION?
Arsenic is a natural occurring element found in soils and groundwater. According to geographic location, concentrations may vary. It’s highly toxic when found in it’s inorganic state and in high concentrations. This element has two forms, or valences (in a natural setting depending on the amount of oxygen available in groundwater):
- Arsenate (As (V)) – usually in more shallow aquifers with higher levels of oxygen.
- Arsenite (As (III)) – usually in deeper, anaerobic ground waters.
In the pH range of 4 to 10, the predominant As (III) compound is neutral in charge, while As (V) is negatively charged.
According to the World Health Organization, long term exposure to arsenic (through drinking contaminated water) can lead to skin lesions, skin cancer or other effects.
WHAT IS ARSENIC REMOVAL?
When arsenic levels are too high, it may be necessary to treat the water to remove it.
Because of As (V)’s negative charge, removal efficiency is greater than that of As (III). In many cases, oxidation of As (III) to convert it to As (V) may be necessary to efficiently remove arsenic from drinking water.
To guarantee high efficiency in filtration, the process starts with Oxidation. Then, Coagulation takes place to group the Arsenic particles. Consequently forcing them through an Ion Exchange filter.
Additionally, Membrane Technologies can be used to eliminate Arsenic. This is done using low pressure membranes, such as micro-filtration, or high pressure, for example Reverse Osmosis.

HOW TO KNOW IF THE WATER IS CONTAMINATED?
Since every water source is unique, we strongly recommend performing a complete water analysis to properly size the arsenic removal solution. These are the basic parameters to know in order to properly size the solution.
- Total dissolved solids (TDS)
- Iron content (Fe)
- Hardness ( Ca and Mg )
- Sulfate (SO4)
- BOD
- Turbidity
- Heavy metals content (Pb ) , (Cd), Cr, (Sb) and (Mo)
- Incoming Power
- Incoming feed flow and source
OUR SOLUTION
Typically we recommend the following steps:
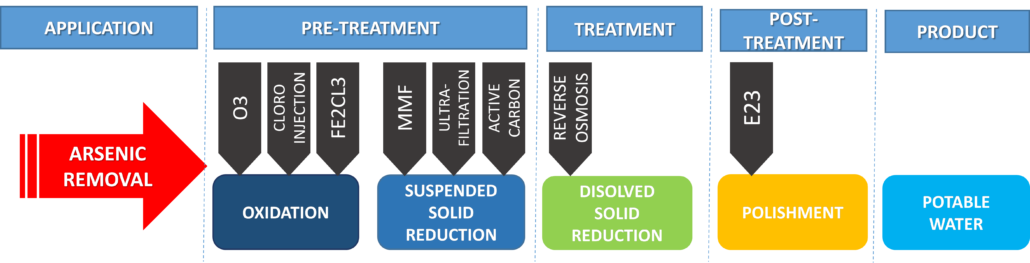
The ADVANCEES system for arsenic removal is shown (see flowchart below). However, this flowchart varies based on raw water quality and the customer’s requirement. Many options are possible, the best solution accommodating the minimum life cycle cost, such as low operating cost, low maintenance and, easy to operate and monitor.
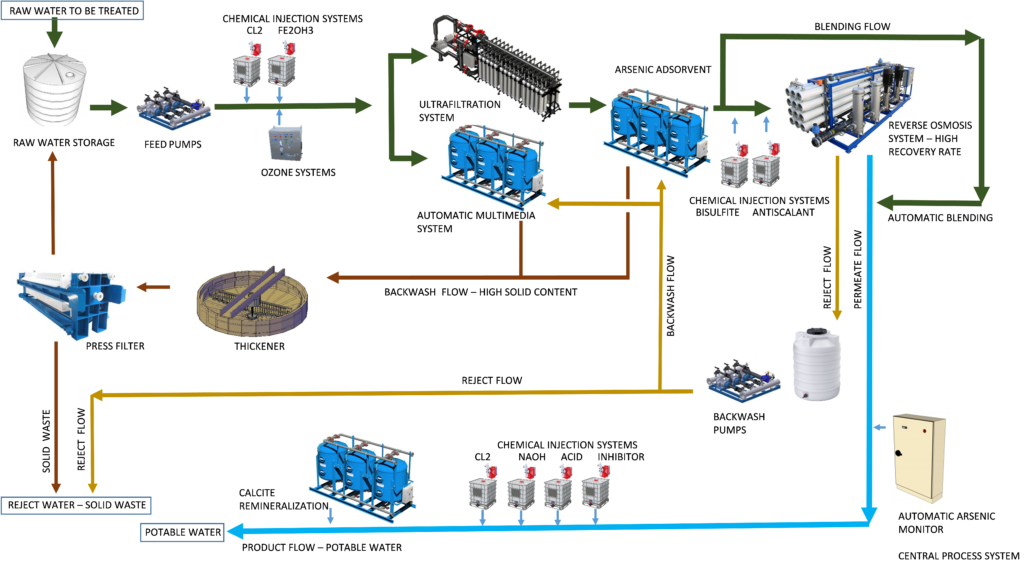
THE BEST ARSENIC REMOVAL PROCESS
Arsenic contamination in water is a critical global issue due to its severe risks to both human health and the environment. Naturally occurring arsenic can leach into groundwater from geological formations, leading to elevated levels in drinking water supplies.
Health Risks of Arsenic Exposure
Long-term exposure to arsenic-contaminated water is linked to:
- Skin disorders and sores
- Bladder, lung, and skin cancers
- Cardiovascular disease
- Neurological complications
The degree of health risk is determined by the concentration and duration of exposure.
How Arsenic Is Removed from Water
Several methods are used to remove arsenic from water, ranging from traditional chemical processes to modern filtration technologies.
Chemical Coagulation and Filtration
Chemicals like alum or ferric chloride are added to water to form flocs that bind to arsenic particles, allowing them to settle and be filtered out. Other filtration media like iron-based media or activated alumina are also effective.
Adsorption & Ion Exchange
Innovative adsorbent materials—such as iron oxides or activated carbon—target and remove arsenic ions. Ion exchange resins replace arsenic with less harmful ions like chloride or sulfate.
Membrane Technologies
Reverse osmosis (RO) and nanofiltration are highly effective, using semi-permeable membranes to physically block arsenic particles while allowing clean water to pass through.
Oxidation
Oxidizing agents like chlorine or potassium permanganate transform arsenic into less harmful forms, making them easier to remove via filtration or sedimentation.
Challenges & Considerations
Cost & Maintenance
Some advanced technologies require significant investment and ongoing maintenance. Energy-intensive systems like RO may raise operational costs and environmental concerns, especially in resource-limited areas.
Environmental Impact
High energy consumption and brine waste from certain systems can impact the environment. Sustainable alternatives such as solar-powered arsenic removal equipment and biological treatment using microorganisms offer more eco-friendly solutions.
Global Impact & Regulatory Compliance
Arsenic contamination affects many regions globally, including Bangladesh, India, China, and parts of Latin America. In these areas, governments and organizations are actively working to improve access to clean water.
Most countries have adopted regulations to limit arsenic in drinking water. These standards are often guided by the World Health Organization (WHO), though specific limits vary by region.
System Performance and Water Quality
- Effectiveness: Technologies can drastically reduce arsenic levels, although complete removal may be influenced by local water chemistry.
- Taste & Odor: Properly designed systems usually do not alter water taste or odor. Minor changes may occur depending on the technology used.
- Maintenance: Regular inspection and servicing ensure system performance, prevent failures, and maintain compliance with safety standards.
