Water Treatment Applications for Industrial & Commercial Needs
At ADVANCEES, we specialize in advanced water treatment solutions tailored to meet the needs of a wide range of industrial and commercial applications. From desalination and arsenic removal to ultrapure water production and deionization, our systems are engineered to deliver reliable performance in challenging environments. Our expertly designed processes cover every stage of treatment—pre-treatment, primary filtration, and post-treatment—to ensure water quality standards are met or exceeded for each unique application.
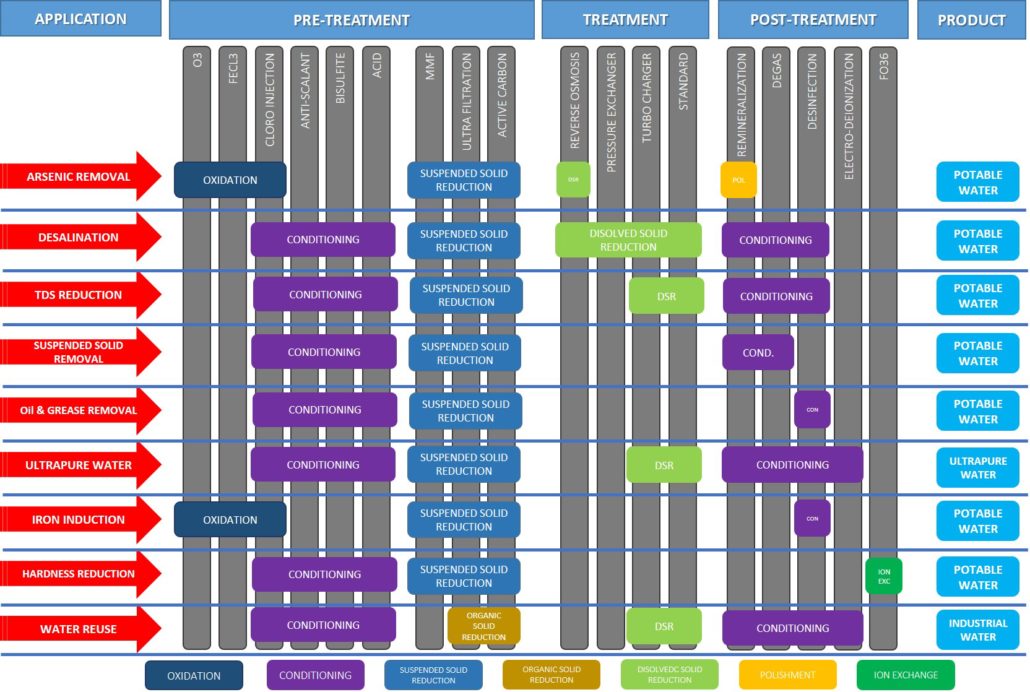
Full Treatment Matrix by Application
The matrix below outlines the recommended pre-treatment, treatment, and post-treatment systems for each water application. It helps ensure that every project is equipped with the right solution from start to finish.
Why Choose ADVANCEES for Your Application Needs?
- Custom-engineered solutions for diverse industry needs
- Skid-mounted, containerized, and solar-powered options available
- Expertise across desalination, filtration, ion exchange, and more
- Proven performance in complex, industrial-grade installations
